Pediatric Cardiac Interventions
Various cardiac interventions can be done in children with congenital heart disease
Diagnostic Cardiac Catheterisation
DEVICE CLOSURES: ASD, PDA, VSD, CORONARY AV FISTULA, PULMONARY AV MALFORMATIONS, AORTO-PULMONARY WINDOW
BALLOON PROCEDURES: BALLOON PULMONARY VALVOTOMY, BALLOON AORTIC VALVOTOMY, COARCTATION BALLOON DILATATION
STENTING: COARCTATION STENTING, BRANCH PULMONARY ARTERY STENTING
ASD DEVICE CLOSURE
Atrial septal defect (ASD) is a form of a congenital heart defect that enables blood flow between two compartments of the heart called the left and right atria. Normally, the right and left atria are separated by a septum called the interatrial septum. If this septum is defective or absent, then oxygen-rich blood can flow directly from the left side of the heart to mix with the oxygen-poor blood.
- Frequent respiratory or lung infections
- Difficulty breathing
- Tiring when feeding (infants)
- Shortness of breath when being active or exercising
- Skipped heartbeats or a sense of feeling the heartbeat
- A heart murmur, or a whooshing sound that can be heard with a stethoscope
- Stroke
- Depending on the site of defect, different types of ASD are
- Ostium Secundum ASD
- Ostium Primum ASD
- Sinus venosus type of defect (SVC type, IVC type)
- Coronary sinus ASD
Ostium secundum ASDs with adequate SVC, mitral, aortic and IVC rims.
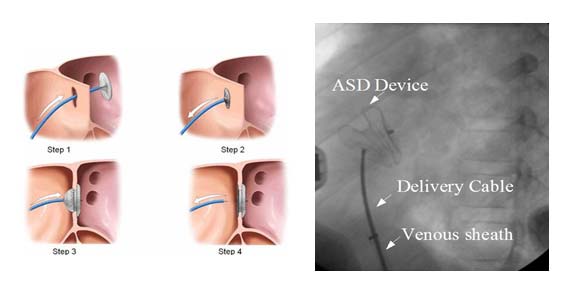
Device closure of ASD is suitable for secondum ASD with a good rim all around for holding the two discs together. Trans-thoracic echo (TTE) is done to assess the superior, aortic and mitral rims. A guide wire is introduced through the femoral vein into the inferior vena cava and further through the right atrium across the ASD. The tip of the wire is placed in the pulmonary vein and a long venous sheath is introduced. Once the sheath is in position, the device attached to the delivery cable is introduced into the sheath under water to avoid air bubbles in the system. Once the device reaches the left atrium, the left atrial disc of the device is released first and brought in contact with the left atrial side of the ASD. When the position is judged ideal, the right atrial disc is allowed to form by withdrawal of the sheath. Once the two discs are in position with the waist across the ASD, slight wiggling is done to make sure that the device is perfectly fitting and has no tendency for dislodgement. Position is confirmed by TEE/ TTE with special care to see that the device does not interfere with the function of the AV valves. Once everything is fine, the device is released by unscrewing the delivery cable.
VSD DEVICE CLOSURE
Ventricular Septal Defect (VSD) is one of the most common congenital heart defects .Before a baby is born, the right and left ventricles of its heart are not separate. As the fetus grows, a wall forms to separate these two ventricles, if the wall does not completely form, a hole remains. This hole is known as a ventricular septal defect, or a VSD.
In most children, the cause isn't known. VSD is a very common type of heart defect. It is sometimes associated with some genetic syndromes like Down’s syndrome, DiGeorge syndrome; chromosomal disorders etc. Some children can have other heart defects along with VSD.
Normally, the left side of the heart only pumps blood to the body, and the heart's right side only pumps blood to the lungs. In a child with VSD, blood can travel across the hole from the left pumping chamber (left ventricle) to the right pumping chamber (right ventricle) and out into the lung arteries. If the VSD is large, the extra blood being pumped into the lung arteries makes the heart and lungs work harder and the lungs can become congested and cause difficulty in feeding and fast breathing, recurrent lung infections(pneumonia).
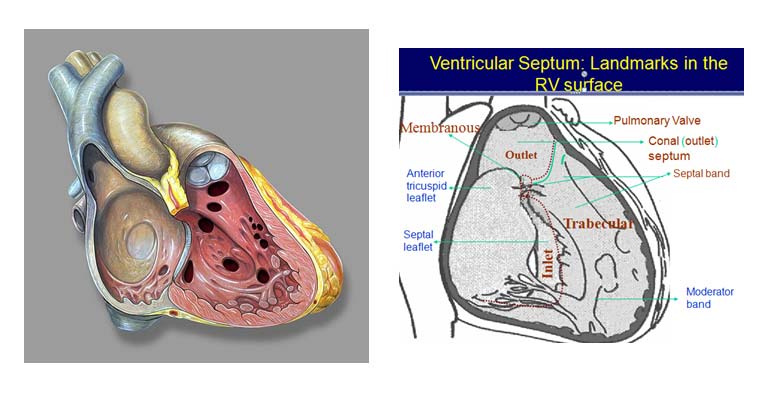
Depending on the location of the defect in the ventricular septum, the various types of VSD are :
- Inlet VSD
- Muscular VSD
- Perimembranous VSD
- Conoventricular VSD (outlet/doubly committed/ subarterial subaortic / subpulomnic VSD)
Spontaneous closure
- 25-30% overall
- 60% close by 3 years and 90% by 8 years’
- 75-80% of small VSDs (Muscular and perimembranous types)
- Rare with large defects
- Inlet VSDs and malalignment VSDs do not close or reduce in size
If the VSD is small, chid is asymptomatic because the heart and lungs don't have to work harder. The only abnormal finding is a loud murmur (noise heard with a stethoscope).
If the VSD is large, the child may breathe faster and harder than normal. Infants may have difficulty in feeding and growing at a normal rate. Symptoms may not occur until 4-6 weeks after birth. High pressure may occur in the blood vessels in the lungs because more blood than normal is being pumped there. Over time this may cause permanent damage to the lung blood vessels.
Timing Guidelines :
Large Perimembranous/Outlet/Inlet VSD
- Uncontrolled CHF: anytime; preferable after 1 month.
- Severe PAH/controlled CHF: Anytime between 3 -6 months
- Age at operation beyond 1 month does not impact surgical outcome
Large Muscular VSD
- Higher likelihood of spontaneous closure
- Close watch for signs of spontaneous closure
- Medical management of CHF may be tried until the age of 6 months
- At times PA banding
Moderate Sized VSDs
- PA pressures 50 – 66% systemic, LA, LV enlarged
- Elective closure between 1 - 2 years
- Earlier if there is failure to thrive or significant LRTI
Small VSDs
- Yearly follow-up for complications like Aortic valve prolapse/AR, RVOTO, SBE.
- Elective intervention if complications develop.
- Small Outlet VSD with RCC prolapse with or without AR –closure by 2-3 years.
Small VSDs often close spontaneously.
There isn't any medicine or other treatment that will make the VSD get smaller or close any faster than it might do naturally.
Moderate sized VSDs do become smaller in size but it has be observed whether it has become smaller due to restriction by any valve of the heart like tricuspid valve or aortic valve.If the VSD is restricted by aortic valve prolapse and causing aortic regurgitation then that VSD needs to be closed even if VSD is smaller in size as aortic valve may get further damage.
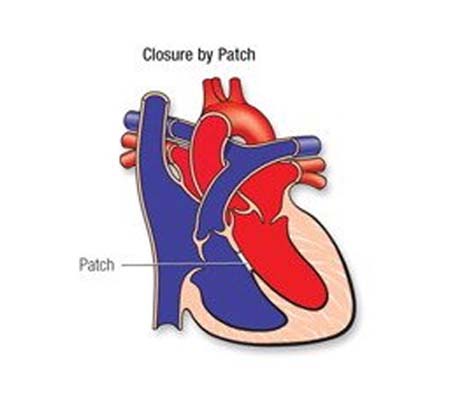
If the VSD is large, open-heart surgery may be needed to close it and prevent serious problems. Babies with VSD may develop severe symptoms and early repair, within the first few months, is often necessary. The repair may be delayed in other babies. Medicines may be used temporarily to help with symptoms, but they don't cure the VSD or prevent permanent damage to the lung arteries.
Some VSDs may not close are : malaligned VSD, large inlet VSD
- PM, Outlet and Inlet VSDs – surgery
- Trans-catheter closure :
- Muscular VSDs (older children, weight > 10 kg)
- Post-operative residual VSD
- Perimembranous VSD
Yes, but only selective VSDs can be closed with device
Such as midmuscular VSD, perimembranous VSD which is far from aortic valve.
PDA DEVICE CLOSURE
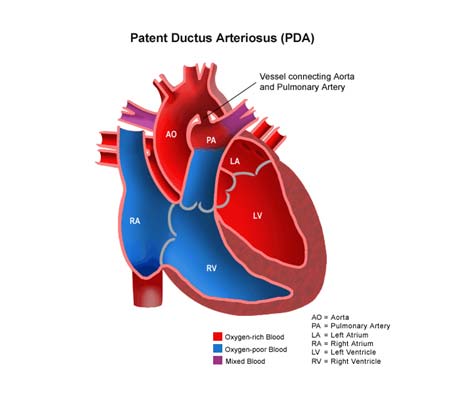
PDA is a common congenital heart defect in which the ductus arteriosus connecting the pulmonary artery and descending aorta persists beyond the first 10 days of life. Patent ductus arteriosus (PDA) is a condition in which the ductus arteriosus does not close. The word "patent" means open.
The ductus arteriosus is a blood vessel that allows blood to go around the baby's lungs before birth. Soon after the infant is born and the lungs fill with air, the ductus arteriosus is no longer needed. It usually closes in a couple of days after birth. If the vessel doesn't close, it is referred to as a PDA.
PDA leads to abnormal blood flow between the aorta and pulmonary artery, two major blood vessels that carry blood from the heart.
- Depending on the size of the PDA, there is variable left-to-right shunt of blood from the aorta to the pulmonary artery. Morbidity and mortality are related to volume of blood flow
- Clinical presentation depends on the size of the PDA; infants with a small PDA are often asymptomatic; those with a larger PDA may have symptoms of heart failure, growth retardation, and may be prone to lower respiratory tract infections. Although present from birth patients may become symptomatic at any age
Incidence and prevalence :
- Incidence is inversely correlated with gestational age; PDA is the most common congenital heart defect in preterm infants
- Approximately 80 cases per 10,000 live births for PDA diagnosed by echocardiography. Incidence may be as low as 6 cases per 10,000 live births for clinically significant PDA
- Up to 60% of infants born at less than 28-weeks' gestation have PDA
- PDA represents 5% to 10% of all congenital heart defects
Demographics :
- Inversely correlated with gestational age. A patent ductus is common in premature infants and much less so among fullterm neonates
- Is three times more common in female than male neonates
- PDA has no racial predilection
- No single genetic cause has been identified, but PDA can be seen in association with genetic disorders such as Char syndrome, trisomy 21 and 18, CHARGE syndrome, cri du chat syndrome, Noonan syndrome, and Holt-Oram syndrome. There is a 2% to 4% increased incidence of PDA in siblings of affected patients
- PDA is more common in infants born at high altitude, reflecting hypoxia as a cause of PDA
- An increased incidence of PDA has been reported in patients of low socioeconomic status, although this most likely reflects a higher incidence of premature birth
- The cause is unknown, but PDA is associated with prematurity, persistent high levels of prostaglandins, and neonatal hypoxia. There is also a strong association between maternal rubella infection in early pregnancy and PDA in the infant
- PDA can be associated with other congenital heart disease and can be an important source of systemic blood flow in infants with specific cardiac abnormalities such as aortic coarctation
- Term infants with a small PDA are asymptomatic, although a heart murmur may be detected on physical examination. In these patients a continuous 'machinery' murmur usually heard most clearly at the left-upper sternal border is characteristic of PDA. Term infants with a large PDA may present after 4 to 6 weeks with feeding difficulties, failure to thrive and tachypnea; there may be signs of congestive heart failure including pulmonary congestion and jugular venous distention.
- Unlike in full-term infants, a PDA in a premature infant often closes spontaneously: 60% within 3 days and 75% within 3 months. Premature infants on the other hand may present with a rough systolic murmur or with no murmur at all
- The cause is unknown, but PDA is associated with prematurity, persistent high levels of prostaglandins, and neonatal hypoxia. There is also a strong association between maternal rubella infection in early pregnancy and PDA in the infant
- PDA can be associated with other congenital heart disease and can be an important source of systemic blood flow in infants with specific cardiac abnormalities such as aortic coarctation
- Term infants with a small PDA are asymptomatic, although a heart murmur may be detected on physical examination. In these patients a continuous 'machinery' murmur usually heard most clearly at the left-upper sternal border is characteristic of PDA. Term infants with a large PDA may present after 4 to 6 weeks with feeding difficulties, failure to thrive, and tachypnea; there may be signs of congestive heart failure including pulmonary congestion and jugular venous distention.
- Unlike in full-term infants, a PDA in a premature infant often closes spontaneously: 60% within 3 days and 75% within 3 months. Premature infants on the other hand may present with a rough systolic murmur or with no murmur at all
Causes :
- The cause(s) of PDA are unknown, but there are several associated factors that may contribute to the etiology including the following:
- Persistently elevated levels of prostaglandins help maintain an open ductus arteriosus. Premature infants have higher levels of circulating prostaglandins, since prostaglandins are metabolized by mature lungs characteristic of infants born at or near term
- Maternal rubella infection in early pregnancy is associated with PDA in up to 85% of infants
- Relative hypoxia as may occur in infants born with immature lungs, at high altitudes, and/or with coexisting congenital cardiac defects. Normally, the sudden increase in oxygen tension at birth causes a decrease in pulmonary vascular resistance and contraction of smooth muscle in the ductus arteriosus resulting in closure
- Maternal rubella infection in early pregnancy is a serious cause
- PDA is associated with serious genetic disorders such as Char syndrome, trisomy 21 and 18, CHARGE syndrome, cri-du-chat syndrome, Noonan syndrome, and Holt-Oram syndrome. These disorder are associated with noncardiac abnormalities as well
Risk Factors :
- Prematurity, particularly infants born below 28-weeks' gestation, due to high levels of circulating prostaglandins and underdeveloped lungs
- Maternal rubella infection in early pregnancy
- Delivery at high altitudes due to relative hypoxia
Associated disorders
Commonly associated with other congenital cardiac disorders
- Also associated with Char syndrome, trisomy 21 and 18, CHARGE syndrome, cri du chat syndrome, Noonan syndrome, and Holt-Oram syndrome
- Large PDA
Treatment options for repairing a patent ductus arteriosus include monitoring, medications, and closure by cardiac catheterization or surgery.
| Large PDA | CHF, PAH | Early closure ( 1-3 months) |
| Moderate PDA | No CHF, mild – moderate PAH, LA, LV enlarged | Closure 3-6 months |
| Small PDA | No PAH, continuous or long systolic murmur | Elective closure after 6 – 12 months. |
| Silent PDA | No murmur, detected on echo | No closure |
PDA device closure, coil closure
- PDA coil closure
- Surgical Closure-PDA ligation
PDA closures – How?
- Vast majority of cases – trans-catheter closure ( coils or device)
- Indications for surgery :
- Very large PDA > 4 mm
- Weight < 3 kg
Very shallow ampulla